Page 223 - Reliance Foundation School Koparkhairane - School Magazine - Zenith 2021-22
P. 223
ORIGIN OF SOAPS we spend a long time selecting one soap? How does one soap differ from the other?
Nowadays soaps available in the market are chemically prepared substances. As
Rakesh was playing on a playground with his the chemical composition varies, the cleansing, texture, and smell of each soap
friends; on returning home he washed his vary from one another.
hands and feet with his favourite soap and
went for eating food. Riya his younger sister Soap chemistry has undergone drastic change post-independence. The growing
was hungry as she was back from her school population and increasing demands have given a boost to the manufacturing
and without washing hands, she sat for industries. Soaps were classified under speciality chemicals. Nowadays we see a
eating food. What difference does it make to variety of soaps like baby soap, perfumed soaps, premium soaps etc. We also have
wash hands before eating food? Cleaning now liquid soaps used for shower wash and handwashing.
hands helps us to keep germs away. Adjacent
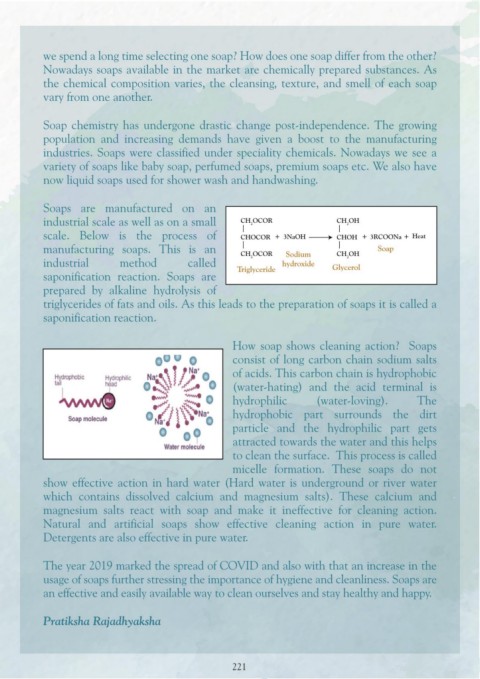
image shares information on how we should wash our hands. Soaps are manufactured on an
industrial scale as well as on a small CH OCOR CH OH
2
2
The image on the left shares when we should wash scale. Below is the process of CHOCOR 3NaOH CHOH 3RCOONa Heat
our hands to keep ourselves germ-free. In the modern manufacturing soaps. This is an CH OCOR Sodium CH OH Soap
2
world of Artificial Intelligence (AI), why has industrial method called 2 hydroxide Glycerol
self-cleanliness suddenly become a priority? Because saponification reaction. Soaps are Triglyceride
of the increase in a variety of diseases spreading prepared by alkaline hydrolysis of
globally. triglycerides of fats and oils. As this leads to the preparation of soaps it is called a
saponification reaction.
When you ask any person around you, “how do we
clean ourselves?” The immediate answer is by using How soap shows cleaning action? Soaps
soap and water. On being asked, “which soap do you consist of long carbon chain sodium salts
use?” The usual answers are Dettol, Lux, Santoor, of acids. This carbon chain is hydrophobic
Dove, etc. few of which are seen below in the image. (water-hating) and the acid terminal is
hydrophilic (water-loving). The
Do you know what are the common ingredients of soap? The common answer is hydrophobic part surrounds the dirt
some essential oils, Sodium hydroxide (NaOH) and aromatic agents. Then why do particle and the hydrophilic part gets
attracted towards the water and this helps
to clean the surface. This process is called
micelle formation. These soaps do not
show effective action in hard water (Hard water is underground or river water
which contains dissolved calcium and magnesium salts). These calcium and
magnesium salts react with soap and make it ineffective for cleaning action.
Natural and artificial soaps show effective cleaning action in pure water.
Detergents are also effective in pure water.
The year 2019 marked the spread of COVID and also with that an increase in the
usage of soaps further stressing the importance of hygiene and cleanliness. Soaps are
an effective and easily available way to clean ourselves and stay healthy and happy.
Pratiksha Rajadhyaksha
220 221